|
| NAME |
Magnesium Stearate |
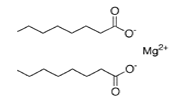
| FORMULA |
Mg(C18 H35 O2)2 |
| Molecular Wt. |
591.27 |
| CAS No. |
557-04-0 |
|
Magnesium stearate, also called octadecanoic acid, magnesium salt, is a white substance which is solid
at room temperature. It has the chemical formula Mg(C18H35O2)2. It is a salt containing two equivalents of
stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate melts at about
120 °C, is not soluble in water, and is generally considered safe for human consumption at levels below
2500 mg/kg per day. In 1979, the FDA's Subcommittee on GRAS (generally recognized as safe)
Substances (SCOGS) reported, "There is no evidence in the available information on ... magnesium
stearate ... that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when
they are used at levels that are now current and in the manner now practiced, or which might reasonably."
|
Application
Magnesium stearate is often used as a diluent in the manufacture of medical tablets, capsules and
powders. In this regard, the substance is also useful, because it has lubricating properties, preventing
ingredients from sticking to manufacturing equipment during the compression of chemical powders into
solid tablets; magnesium stearate is the most commonly used lubricant for tablets. Studies have shown
that magnesium stearate may affect the release time of the active ingredients in tablets, etc., but not that
it reduces the overall bioavailability of those ingredients. As a food additive or pharmaceutical excipient,
its E number is E470b.
Magnesium stearate is also used to bind sugar in hard candies and is a common ingredient in baby
formulas. In pure powder form, the substance can be a dust explosion hazard, although this issue is
effectively insignificant beyond the manufacturing plants using it.
Magnesium stearate is manufactured from both animal and vegetable oils. Some nutritional supplements
specify that the magnesium stearate used is sourced from vegetables.
Magnesium stearate is a major component of "bathtub rings." When produced by soap and hard water,
magnesium stearate and calcium stearate both form a white solid insoluble in water, and are collectively
known as "soap scum."
|
| Packing |
25 kgs HDPE Bags with Liner inside. |
|
|